Your Oecd guidelines for animal studies images are ready in this website. Oecd guidelines for animal studies are a topic that is being searched for and liked by netizens now. You can Find and Download the Oecd guidelines for animal studies files here. Download all royalty-free images.
If you’re searching for oecd guidelines for animal studies images information linked to the oecd guidelines for animal studies interest, you have pay a visit to the ideal site. Our website always gives you suggestions for refferencing the maximum quality video and picture content, please kindly surf and locate more informative video articles and images that fit your interests.
Oecd Guidelines For Animal Studies. They were first published in 1981. Each dose group and concurrent control group should contain at least 50 animals of each sex. Regulatory acceptance of in vitro studies ☆ This is a new type of oecd guideline that uses several types of combined information to provide chemical safety information and can replace the need for animal test data.
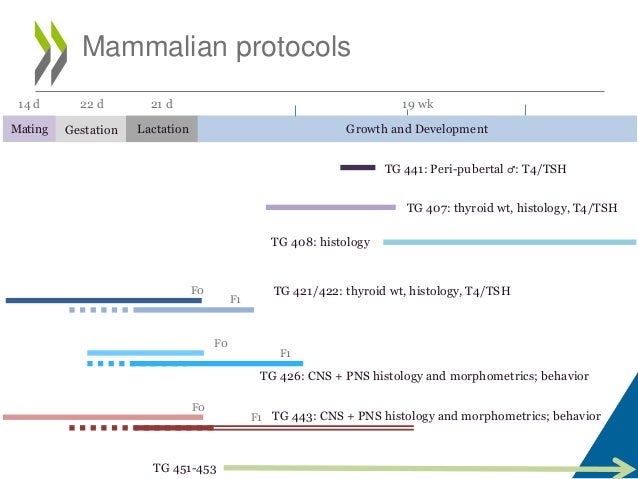 Overview of OECD Test Guidelines for Thyroid Effects From slideshare.net
Overview of OECD Test Guidelines for Thyroid Effects From slideshare.net
This is a new type of oecd guideline that uses several types of combined information to provide chemical safety information and can replace the need for animal test data. Gives information on health hazards. Each dose group and concurrent control group should contain at least 50 animals of each sex. They are split into five sections: Journal of natural s ciences. Three alternative test methods (guidelines 420, 423,and 425) to the.
According to maurice whelan, head of eurl ecvam “this guideline paves the way to the adoption of more harmonised approaches that address regulatory needs and reduce the use of animal studies.
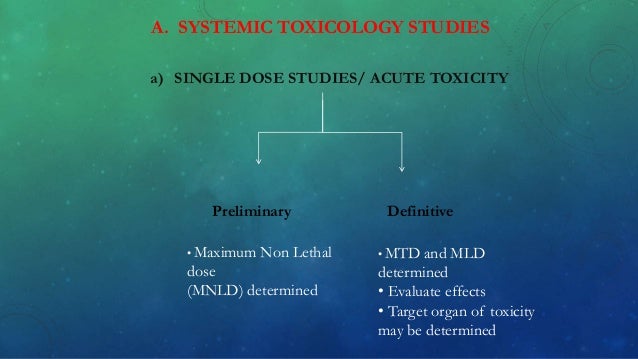
May provide indications for the need for additional studies. Acute oral toxicity oecd guidelines no.401 (conventional acute toxicity method ) in a study of toxic characteristics of substance, acute oral toxicity testing is initial step. Data and research on test guidelines including chemical testing and assessment, chemical safety, animal welfare, endocrine disrupters, good laboratory practice (glp), mutual acceptance of data (mad)., over 25 years ago, the oecd recognised the need to protect animals in general and in particular those used in experimental work. They were first published in 1981. They are split into five sections: The original guideline 452 was adopted in 1981.
 Source: slideshare.net
Source: slideshare.net
Guidelines for humane endpoints in animal study protocols general • unrelieved pain and distress must be avoided whenever possible. The project to develop this new guideline started in 2017 and was led by the united states, the european commission joint research centre and health canada, supported by a group of nominated experts. Further groups of animals may be dosed at higher or lower fixed doses, depending on the presence or absence of signs of toxicity or mortality. Guidelines for humane endpoints in animal study protocols general • unrelieved pain and distress must be avoided whenever possible. Journal of natural s ciences.
 Source: slideshare.net
Source: slideshare.net
The project to develop this new guideline started in 2017 and was led by the united states, the european commission joint research centre and health canada, supported by a group of nominated experts. One dose used per group. Detailed guidance on and discussion of the principles of dose selection for chronic toxicity The project to develop this new guideline started in 2017 and was led by the united states, the european commission joint research centre and health canada, supported by a group of nominated experts. According to maurice whelan, head of eurl ecvam “this guideline paves the way to the adoption of more harmonised approaches that address regulatory needs and reduce the use of animal studies.
 Source: uxunul1.notepad.ru.net
Source: uxunul1.notepad.ru.net
Further groups of animals may be dosed at higher or lower fixed doses, depending on the presence or absence of signs of toxicity or mortality. Data and research on test guidelines including chemical testing and assessment, chemical safety, animal welfare, endocrine disrupters, good laboratory practice (glp), mutual acceptance of data (mad)., over 25 years ago, the oecd recognised the need to protect animals in general and in particular those used in experimental work. One dose used per group. Nonclinical evaluation of drug safety usually consists of standard animal toxicology studies. The guidelines are elaborated with the assistance of experts from regulatory agencies, academia, industry, environmental and animal welfare organisations.
 Source: syntechresearch.com
Source: syntechresearch.com
They are split into five sections: Nonclinical evaluation of drug safety usually consists of standard animal toxicology studies. • humane endpoints are defined as the point at which pain or distress is prevented, This is a new type of oecd guideline that uses several types of combined information to provide chemical safety information and can replace the need for animal test data. Outlined in the oecd guidance document on the recognition, assessment, and use of clinical signs as humane endpoints for experimental animals used in safety evaluation (16), in particular paragraph 62 thereof, should always be followed 7.
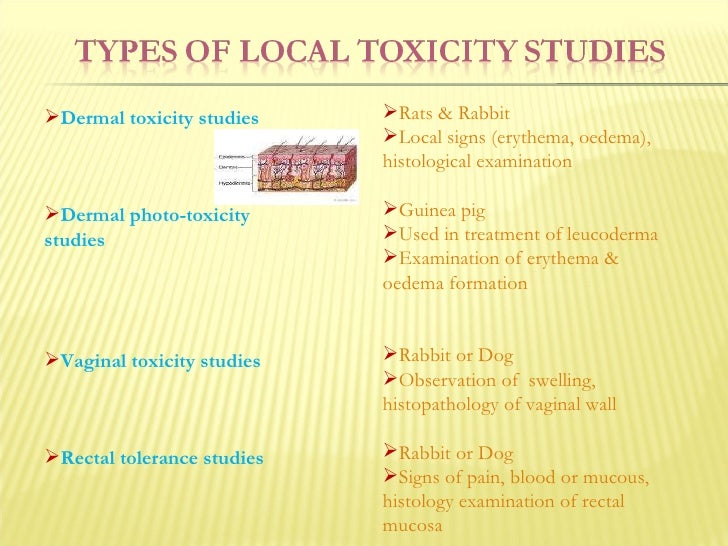 Source: slideshare.net
Source: slideshare.net
May provide indications for the need for additional studies. Gives information on health hazards. Physical chemical properties section 2: Nonclinical evaluation of drug safety usually consists of standard animal toxicology studies. • humane endpoints are defined as the point at which pain or distress is prevented,
 Source: adpen.com
Source: adpen.com
The guidelines are elaborated with the assistance of experts from regulatory agencies, academia, industry, environmental and animal welfare organisations. O a combined chronic toxicity/carcinogenicity study all three of them based on oecd guidelines for the testing animal studies with whole food and development (oecd) guidelines for oral acute toxicity, in sub chronic toxicity study, there was a. Test substance administered orally, in graduated doses to several groups of experimental animals. • requirements for the storage and retrieval of records and data 8. Outlined in the oecd guidance document on the recognition, assessment, and use of clinical signs as humane endpoints for experimental animals used in safety evaluation (16), in particular paragraph 62 thereof, should always be followed 7.
 Source: slideshare.net
Source: slideshare.net
The project to develop this new guideline started in 2017 and was led by the united states, the european commission joint research centre and health canada, supported by a group of nominated experts. Outlined in the oecd guidance document on the recognition, assessment, and use of clinical signs as humane endpoints for experimental animals used in safety evaluation (16), in particular paragraph 62 thereof, should always be followed 7. This procedure continues until the dose causing evident toxicity or no more than one death is identified, or when no effects are seen at the What’s more, data generated under this guideline must be mutually accepted by all 36 oecd member countries. Detailed guidance on and discussion of the principles of dose selection for chronic toxicity
 Source: slideshare.net
Source: slideshare.net
The project to develop this new guideline started in 2017 and was led by the united states, the european commission joint research centre and health canada, supported by a group of nominated experts. • humane endpoints are defined as the point at which pain or distress is prevented, One dose used per group. Data and research on test guidelines including chemical testing and assessment, chemical safety, animal welfare, endocrine disrupters, good laboratory practice (glp), mutual acceptance of data (mad)., over 25 years ago, the oecd recognised the need to protect animals in general and in particular those used in experimental work. The project to develop this new guideline started in 2017 and was led by the united states, the european commission joint research centre and health canada, supported by a group of nominated experts.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title oecd guidelines for animal studies by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.